| |
Some Phase Diagrams |
 |
Here are two phase diagrams for illustration purposes. I've chosen the diagrams
for two old acquaintances: - The system copper (Cu) - tin (Sn)
- The system copper (Cu) - zinc (Zn)
Let's start with copper (Cu) - tin (Sn). This system contains what we call "bronze".
Here is the phase diagram: |
| |
|
|
|
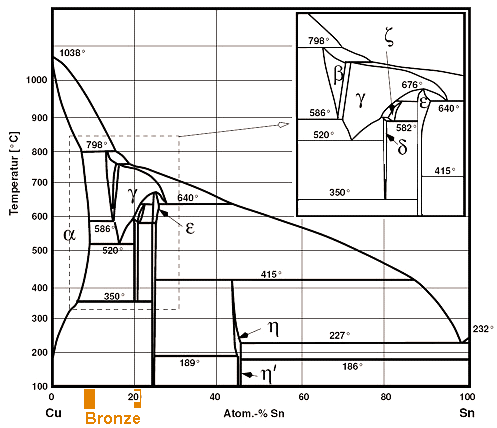
| | Phase diagram of copper (Cu) and tin (Sn) |
| The colored regions show typical bronze compositions. |
|
| |
|
|
 |
That doesn't look so simple, especially at the copper-rich side where we will find the "common"
bronzes as indicated. It is definitely more complicated than the iron -carbon phase diagram that exercised us so much already.
|
 |
Here is the copper (Cu) - zinc (Zn) phase diagram. This system contains what we
call "brass". |
|
| |
| |
 |
| Phase diagram of copper (Cu) and zinc (Zn). |
| The colored regions show typical brass compositions. |
|
| | |
|
|
 |
The copper - zinc phase diagram is a bit simpler than the copper - tin phase diagram but still
complex enough. There are all kinds of brass'
but typically we are at the copper-rich side. |
 |
Of course, if we want to look at all copper
alloys, we would need a bunch of more binary phase diagrams, in particular for the elements arsenic (As), antimony, (Sb),
silver (Ag), and lead (Pb) since these are often found in antique "bronzes". For modern "bronzes" we
need to consider at least aluminum (Al), beryllium (Be), silicon (Si), phosphorus (P) and manganese (Mn). |
|
 |
If we go for the next step and look at real copper alloys
- antique or modern - we need to consider phase diagram with more than two components, and then things get really complicated. |
 |
There are many names for copper alloys, and they are mostly confusing and mixed
up. We have two general names: - Bronze
One tends to call a copper-alloy "bronze" if it looks reddish-brown, no matter what it consists of.
- Brass
One tends to call a copper-alloy "brass" if it looks golden-yellowish,
no matter what it consists of. |
|
 |
Being a bit more specific, one might add a qualifier, for examples arsenic or phosphorus bronze,
bell bronze or bell metal (high tin content), and so on.
Then there are a large number of names for all kinds of modifications
that seem to be very culture dependent (there are no good translations). "German silver" (47–64 % Kupfer,
10–25 % Nickel, 15–42 % Zink), for example, is "Neusilber" (new silver) in German, and so on. |
 |
I will have more
to say about bronze later on. Here we only consider how Young's modulus changes with increasing tin concentration. If
what I claimed before is right, Young's modulus should change
smoothly between the copper value and the tin value if the composition changes from pure copper to pur tin. |
| | |
|
 |
| Young's modules for copper - tin alloys |
| The composition for major phases is indicated in color. |
|
| | |
|
 |
Well - it almost does. There is, however, a big deviation
from the weighted average around 20 % tin. This is clear, however. A very complex intermetallic compound is formed at this
concentrations with a Young's modulus that has nothing to do with that of copper (Cu) or tin (Sn).
In other words: The
rule of "smooth" property changes only applies to system where there is no radical change in the phases. |
© H. Föll (Iron, Steel and Swords script)



